Additional information is available on The Joint Commission Website:
https://www.jointcommission.org/what-we-offer/certification/certifications-by-setting/
https://www.jointcommission.org/what-we-offer/certification/certifications-by-setting/
Contact your Certification Account Executive if you have additional questions.Topics Covered in this section:
Certification Basics
ACPC and using eCQMs for Certification
Certification Resources
...
| Panel |
|---|
| panelIconId | atlassian-info |
|---|
| panelIcon | :info: |
|---|
| bgColor | #B3D4FF |
|---|
|
Certification Basics |
| Expand |
|---|
| title | What are the deadlines for certification data submission? |
|---|
|
1Q certification data is due June 30th
2Q certification data is due September 30th
3Q certification data is due December 31st
4Q certification data is due March 31st |
...
| Expand |
|---|
| title | If a hospital reports the PC chart-abstracted measures for ORYX accreditation reporting purposes, can the hospital utilize an ORYX vendor to submit their PC data for Perinatal Care certification purposes? |
|---|
|
Effective 1/1/2020, The Joint Commission no longer has contracts with ORYX chart-abstracted vendors
for certification or accreditation purposes.
Effective 1/1/2020 patient discharges, all hospitals in the Perinatal Care (PNC) certification program
that have been using an ORYX chart-abstracted vendor must manually enter their aggregate data on the
Certification Measure Information Process (CMIP) application available on JC Connect. Hospitals may
use a vendor to assist in data collection and aggregation.
Note: For reporting of the chart-abstracted PC measures for ORYX accreditation reporting purposes,
hospitals submit data via the The Joint Commission’s Direct Data Submission Platform (DDSP). |
| Expand |
|---|
| title | For certification purposes, can we use eCQMs? | Currently |
|---|
| , The Joint Commission is not utilizing eCQMs for certification program purposes. Data must be
reported on the standardized | - | measures and entered into the CMIP application via Joint
Commission Connect® site.
| Expand |
|---|
| title | Currently chart abstracted data for accreditation must be manually entered on the DDSP. Where is chart abstracted data entered for certification? |
|---|
|
Certification data is entered into the Certification Measure Information Process (CMIP) application
(available via Joint Commission Connect® site). |
...
| Expand |
|---|
| title | Can a hospital make corrections to their previously entered CMIP data when data entry errors have been identified? |
|---|
|
Based on the current date, the data for the previous 24 months can be entered/modified.
Data older than 24 months will be set to read-only and health care organizations will not be able to
modify the respective rows. |
| Panel |
|---|
| panelIconId | atlassian-info |
|---|
| panelIcon | :info: |
|---|
| bgColor | #ABF5D1 |
|---|
|
ACPC and using eCQMs for Certification |
| Expand |
|---|
| title | For certification purposes, can we use eCQMs? |
|---|
|
Currently, for ACPC only, eCQM data can be manually entered into CMIP. For all other certification program purposes, chart-abstracted data must be reported on the standardized chart-abstracted measures and entered into the CMIP application via Joint Commission Connect® site. |
| Expand |
|---|
| title | Which measures can an organization submit the electronic clinical quality measure (eCQM) version for ACPC? |
|---|
|
Any of the Perinatal Care required measures can be submitted as either the chart-abstracted (CAM) or eCQM version of the measure to meet ACPC performance measure requirements. PC-01 or ePC-01 Elective Delivery PC-02 or ePC-02 Cesarean Birth PC-05 or ePC-05 Exclusive Human Milk Feeding PC-06 or ePC-06 Unexpected Complications in Term Newborns |
| Expand |
|---|
| title | Are there any considerations organizations should be aware of when selecting which measure version to submit? |
|---|
|
PC-02 and PC-06 (severe rate only) have threshold requirements that must be met for ACPC (see certification manual for details). Organizations should take this into consideration when deciding which measure version to submit. It is important for organizations to have a process in place to ensure the eCQM data is accurate and valid. Some organizations choose to collect both the chart-abstracted version and the eCQM version to compare results. |
| Expand |
|---|
| title | Can organizations submit a combination of chart-abstracted (CAM) and eCQM measures? |
|---|
|
Yes, organizations can submit all chart-abstracted (CAM) PC measures, all eCQM PC measures or a combination of both. Organizations must submit the same version of the measure for the entire year. |
| Expand |
|---|
| title | How often will data need to be submitted? |
|---|
|
Data is required to be submitted quarterly into CMIP for both chart-abstracted (CAM) and eCQM measures for ACPC. Refer to the certification manual for details. |
| Expand |
|---|
| title | Can TJC transfer an organization’s data from DDSP to CMIP? |
|---|
|
No, this functionality is not available. Organizations choosing to submit aggregate data from eCQM measures must enter the aggregate counts manually into CMIP. |
| Expand |
|---|
| title | Can organizations use internal reports for data submission? |
|---|
|
Internal reports may be used to report monthly eCQM performance in CMIP. Data in CMIP are reported by month on a quarterly basis while eCQM data are reported to DDSP by quarter on an annual basis. Currently, the DDSP does not provide reports showing measure performance by month. Enhancements will be made to the DDSP eCQM reports to show monthly counts. Until such time, organizations can use internal data reports to report monthly eCQM performance; however, they should have a means of verifying accuracy of data being submitted. Remember, for ACPC, quarterly data submission is required. |
| Expand |
|---|
| title | How do organizations ensure they are submitting the correct version of the measure? |
|---|
|
In CMIP, choose either the chart-abstracted (CAM) or eCQM version from the drop-down menu for each measure. Complete the zero-attestation for the version NOT submitted. For example, if you select the CAM version of PC-01, you will need to check the zero-attestation box for the eCQM ePC-01 measure. 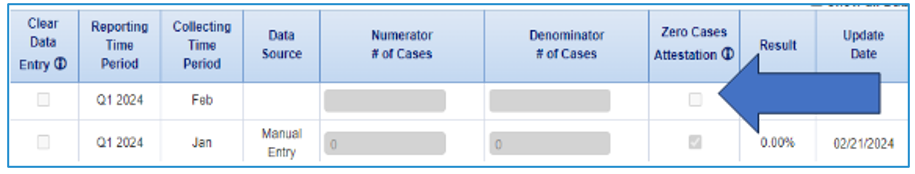 Image Added Image Added |
| Expand |
|---|
| title | What if an organization has multiple sites under the same HCOID number that have ACPC? |
|---|
|
Organizations must have a way to separate their data prior to submission. Data for ACPC must be from individual certified sites. |
| Expand |
|---|
| title | Will data from CAM and eCQM versions be reported together? |
|---|
|
CAM and eCQM data will be reported separately. Once there are enough sites submitting data on each measure version, reports will include comparison data. |
| Expand |
|---|
| title | Why do all Perinatal Care measure versions appear on Tab 6 PM data reports in CMIP? |
|---|
|
Due to how Tab 6 is populated in CMIP, all Perinatal Care measures are listed and need to be addressed. Measures for which the organization submitted data should be completed. Measures which have zero-attestation (no data submitted) will need to have NA entered into the fields. |
| Panel |
|---|
| panelIconId | atlassian-info |
|---|
| panelIcon | :info: |
|---|
| bgColor | #C0B6F2 |
|---|
|
Certification Resources |
| Expand |
|---|
| title | What if I have additional measure related questions? |
|---|
|
Please submit any measure questions to the Question Forum on our website at: https://manual.jointcommission.org . |
| Expand |
|---|
| title | What if I have additional certification related questions? |
|---|
|
Contact your Certification Account Executive if you have additional questions. |
